Day 28
Context
Molecules stay together because they share electrons. Bonds between atoms that are due to sharing electrons are called covalent bonds. Since different atoms have different abilities to attract electrons, sometimes the pair of shared electrons are evenly shared, other times they are not.
Explanation
| Video Links |
Video Popup
|
Class Video
|
Nonpolar molecules are symmetrical and don't have a positive or negative end to the molecule. Polar molecules are not symmetrical and do have a partial negative charge on one end and a partially negative charge on the other end.
After this class you should be able to:
- Define the terms temporary and permanent dipole.
- Explain the difference between polar and nonpolar molecules.
- Identify simple molecules as either polar or nonpolar.
Dipoles
When we talked about a charged rod next to a pith ball we introduced the idea of a dipole. In that case the charged rod caused the electrons in the pith ball to move to one side. This created a temporarily positive side and a temporarily negative side separated across the pith ball. When the rod is taken away the electrons go back to random and there is no separation of charge. Creating the charge on the rod was done using friction. We scrape electrons off of one object onto another. The question now is "How can we create a charge without friction?"
Electronegativity
Electronegativity is a word for how hard an atom can pull on an electron within a bond. Each element has its own amount of pull on those electrons. Some attract more than others. We already know this. Chlorine has a greater attraction for electrons than sodium. In fact chlorine takes the electron away from sodium when it gets the opportunity. These are not polar or nonpolar, they are ionic. We will only be looking at cases where the electrons are shared, not transferred. If they move within a shared bond we say that they are shifted from one side to another. The bigger the difference in electronegativity, the farther the electrons are shifted.
To have a sense of which elements have stronger electronegativity we will identify flourine, F, as the most electronegative element. Whenever flourine competes for an electron it wins. The electrons are shifted toward the flourine atom. It turns out that elements that are closer to flourine on the periodic table have more electronegativity. Oxygen would have a stronger pull on electrons than carbon, etc.
Determining Polarity
It is easy to determine if a diatomic molecule is polar or not. If both atom are the same element it is nonpolar, if they are different elements, it is polar. F2, O2, etc. are nonpolar. CO, carbon monoxide is polar. The oxygen is closer to flourine, so the shared electrons will be closer to the oxygen atom. The electrons have shifted to one side, creating a dipole. This is a permanent dipole because the electrons will always go toward the oxygen in this case. The shift of the electrons is due to the difference in electronegativity of the two atoms. No friction needed!
If there are more than two atoms we will go to looking at symmetry. If the molecule is symmetrical we will assume it is nonpolar. If it isn't symmetrical we will assume it to be polar.
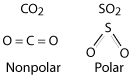
Homework
The homework associated with Day 28 is on Canvas.